A relentless, degenerative neuromuscular disease6
Dystrophin is a large protein found in muscle fibers throughout the body and plays a key structural role in maintaining the integrity of the sarcolemma during normal muscle contraction.3
With little or no dystrophin, people with Duchenne experience progressive and irreversible muscle wasting beginning at birth and continuing throughout life, with motor function worsening over time and leading to premature death.3,7,8

Duchenne is a heterogeneous disease; every child progresses differently, and treatment experience will vary based on individual differences.9
Treatment intervention is critical for people with Duchenne regardless of age or magnitude.7 Even small amounts of endogenous dystrophin (<0.5%) have been shown to make a difference in critical functional parameters.10
Untreated patients experience a persistent loss of function
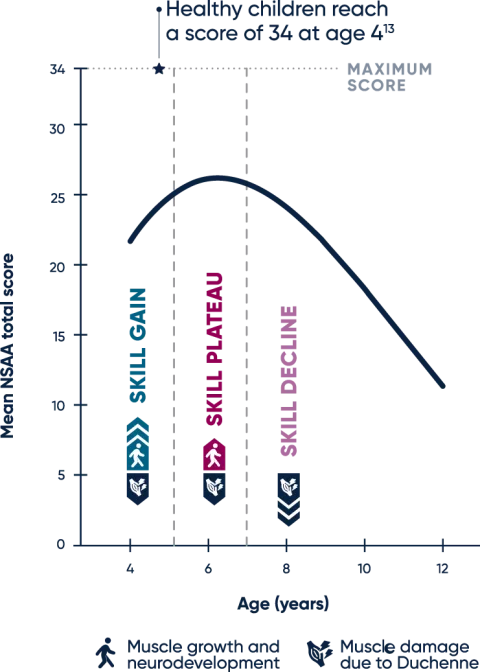
In the natural history of Duchenne, delays in motor development are present in early childhood. At younger ages, muscle growth and neurodevelopment mask decline, so during this time, observed increases or stabilization in motor function scores may conceal muscle degeneration.3,6
Boys with Duchenne typically demonstrate a peak NSAA score of 26 points around 6 years of age. From that point on, muscle loss has a more visible impact on daily function.12
Duchenne natural history: fitted mean trajectory
-
SKILL GAIN
Muscle growth and neurodevelopment drive an increase in NSAA score at younger ages, masking muscle health decline in children with Duchenne6,12
-
SKILL PLATEAU
NSAA score generally peaks at a mean of 26 points at age 6.3 years. At this stage, the underlying disease process offsets skill gains made via muscle growth and neurodevelopment12,13
-
SKILL DECLINE
Increasing fibrosis and muscle loss lead to children with Duchenne losing ambulation between 10 and 13 years of age12,14,15
The NSAA scale is composed of 17 items and helps evaluate changes in gross motor ability over time. The aggregate score is based on the ability to perform a task or skill, such as rise from floor, ascend stairs, or walk, with scores of 0, 1, or 2 representing inability, ability with assistance, and complete independence, respectively.12
NSAA=North Star Ambulatory Assessment.
Timed function tests are early predictors of functional decline16
Untreated patients typically experience a decline on highly sensitive timed function tests (TFTs), which are often able to detect changes in motor function before aggregate measures.16-19
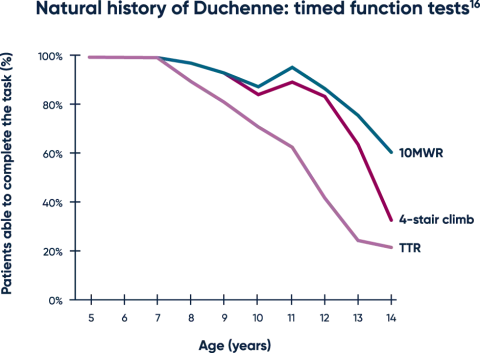
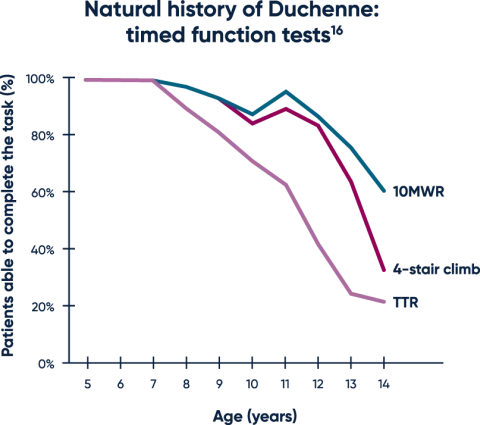
Both TTR and the 10MWR tests are strongly predictive of loss of ambulation, with key thresholds of >5 seconds and >10 seconds, respectively.20,21
MWR=meter walk/run; TTR=time to rise.