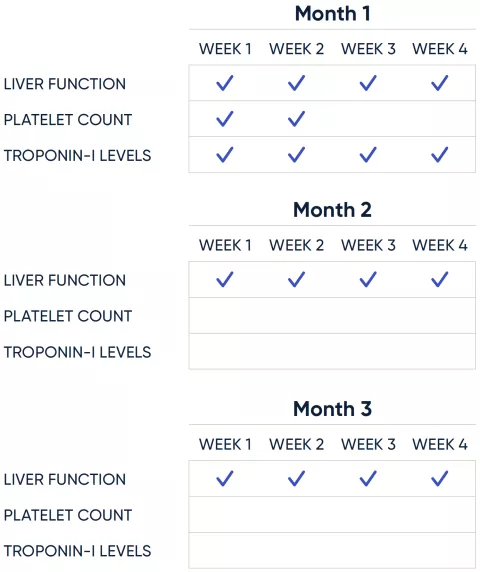
Required monitoring
Weekly safety monitoring is required for at least 3 months (or longer, if clinically indicated) to assess liver function, platelet count, and troponin-I levels.1

Safety considerations1
Adverse reactions typically seen within the first 2 weeks following infusion include nausea, vomiting, thrombocytopenia, and pyrexia. Vomiting may occur as early as on the day of the infusion.
Adverse reactions occurring within the first 2 months include immune-mediated myositis and liver injury.
Infusion-related reactions, including hypersensitivity reactions and anaphylaxis, have occurred during or up to several hours following ELEVIDYS administration. Closely monitor patients during and for at least 3 hours after the infusion, and slow/stop the infusion to administer treatment, as needed.
See Important Safety Information for additional safety considerations when infusing ELEVIDYS.
Postinfusion assessments1

Liver function
- Acute serious liver injury has been observed with ELEVIDYS. Administration of ELEVIDYS may result in elevations of liver enzymes (eg, GGT, ALT) and total bilirubin, typically seen within 8 weeks
- Monitor liver function weekly for the first 3 months via clinical exam and analysis of GGT and total bilirubin levels. Continue monitoring if clinically indicated, until results are unremarkable (normal clinical exam and GGT and total bilirubin levels return to near baseline levels)

Platelet count
- Transient, mild, asymptomatic decrease in platelet count has been observed following ELEVIDYS administration
- Monitor platelet counts weekly for the first 2 weeks
Myocarditis
- Acute serious myocarditis and troponin-I elevations have been observed following ELEVIDYS infusion in clinical trials
- If a patient experiences myocarditis, those with preexisting left ventricle ejection fraction (LVEF) impairment may be at higher risk of adverse outcomes. Patients with moderate to severe LVEF impairment have not been studied in clinical trials with ELEVIDYS
- Monitor troponin-I weekly for the first month. More frequent monitoring may be required in the presence of cardiac symptoms, such as chest pain or shortness of breath
Continue monitoring after the recommended schedule if clinically indicated.
ALT=alanine transaminase; GGT=gamma-glutamyl transferase.
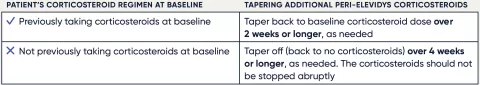
Continuing the required corticosteroid regimen1
Immune responses to the AAVrh74 vector can occur after administration of ELEVIDYS. To reduce the risk associated with an immune response, patients must continue treatment with systemic corticosteroids for at least 60 days after the infusion, unless earlier tapering is clinically indicated.

Recommended corticosteroid regimen dose modification for liver function abnormalities following ELEVIDYS infusion*
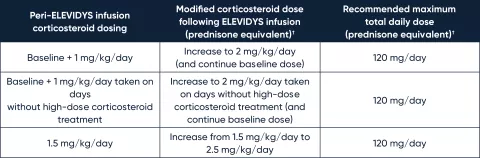
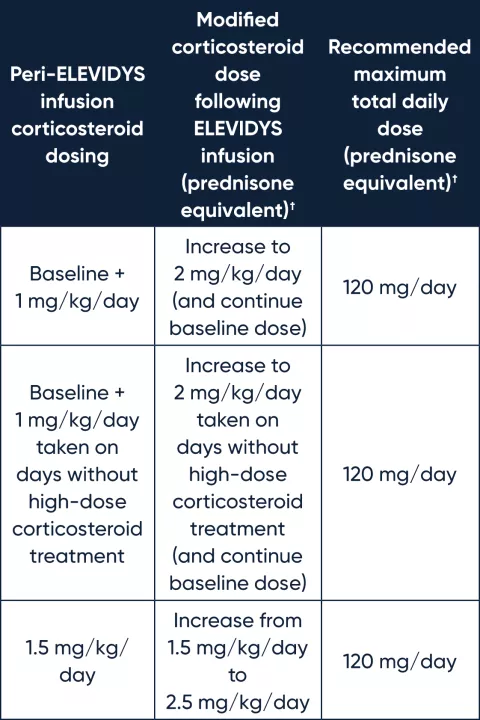
* GGT≥150 U/L and/or other clinically significant liver function abnormalities (eg, total bilirubin >2 × ULN) following infusion. For GGT or bilirubin elevations that do not respond to these oral corticosteroid increases, IV bolus corticosteroids may be considered.
† Corticosteroids other than prednisone and prednisolone have not been studied for use as a peri-ELEVIDYS infusion corticosteroid.
It is critical that patients adhere to a pre- and postinfusion corticosteroid regimen to reduce the risk of an adverse immune response.1

Postinfusion guidance for caregivers1
Counsel patients and caregivers on what to expect postinfusion and reiterate the importance of compliance with the required corticosteroids and monitoring schedule.
-
Speak with your patients or caregivers about the risk of infusion-related reactions,
acute serious liver injury, immune-mediated myositis, and myocarditisAdvise patients and caregivers to contact you immediately if:
- The patient experiences a fast heart rate, fast breathing, swollen lips, being short of breath, nostrils widening, hives, red and blotchy skin, itchy or inflamed lips, rash, vomiting, nausea, chills, or fever, as these may be signs of an infusion-related reaction
- The patient’s skin and/or whites of the eyes appear yellowish, or if the patient misses a dose of corticosteroid or vomits it up
- The patient experiences any unexplained increased muscle pain, tenderness, or weakness, including difficulty swallowing, difficulty breathing, or difficulty speaking, as these may be symptoms of myositis
- The patient begins to experience chest pain and/or shortness of breath, as this may be a sign of myocarditis
-
Remind patients and caregivers about the importance of corticosteroid treatment to help reduce the risk of an immune response to the ELEVIDYS vector (AAVrh74)
-
Reinforce the importance of the weekly monitoring of liver function, troponin-I levels, and platelet counts
-
Advise patients and caregivers that an infection (eg, cold, flu, gastroenteritis, otitis media, bronchiolitis, etc) before or after infusion could lead to more serious complications. Remind them to contact a healthcare provider immediately if symptoms suggestive of infection are observed (eg, coughing, wheezing, sneezing, runny nose, sore throat, or fever)
-
Remind patients, caregivers, and family members about proper hand hygiene when they come into direct contact with the patient’s body waste. Note that they should place potentially contaminated materials in a sealable bag and dispose of it in the regular trash. Encourage this behavior for at least 1 month after infusion to avoid vector shedding