Meaningful treatment benefit in ambulatory patients1
Statistically significant and highly expressed ELEVIDYS micro-dystrophin in treated patients1
Clinical trials confirmed targeted ELEVIDYS micro-dystrophin expression in skeletal muscle cells across ambulation status and ages studied.
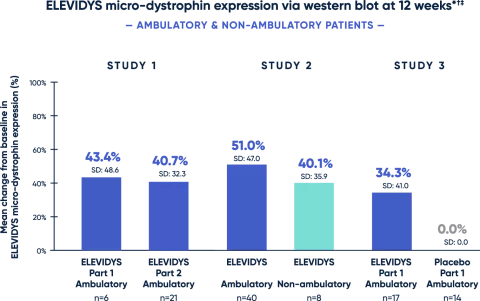
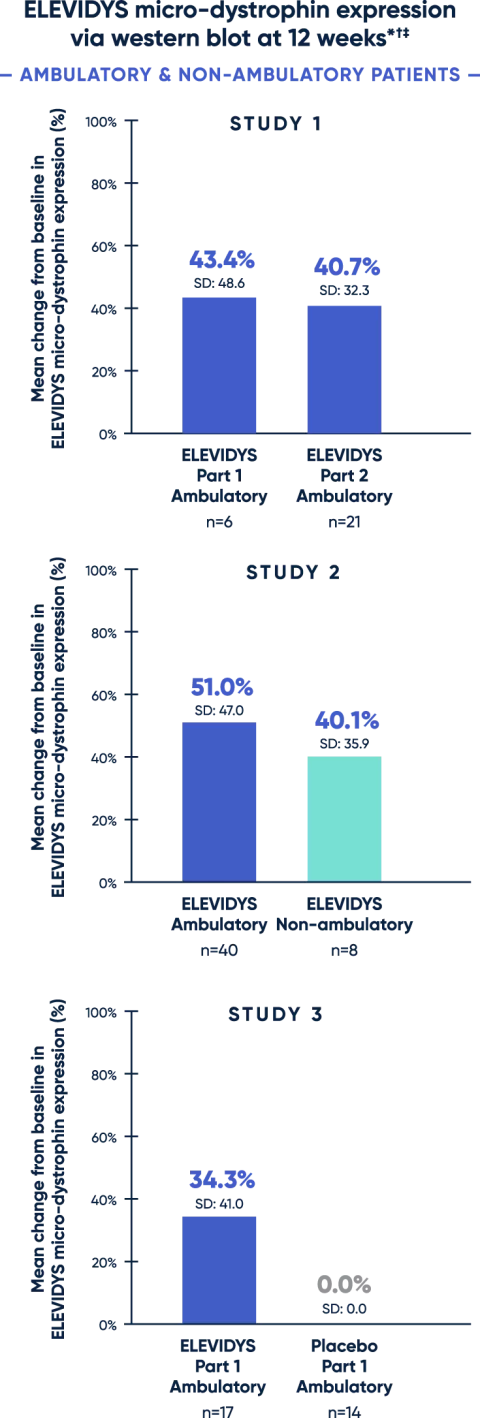
* All patients received 1.33 x 1014 vg/kg, as measured by ddPCR.
† Change from baseline was statistically significant.
‡ Adjusted for muscle content. Control was level of wild-type (normal) dystrophin in normal muscle.
ddPCR=droplet digital polymerase chain reaction.
Patients with Duchenne typically have little to no endogenous dystrophin.3
A clear association between Week 12 micro-dystrophin expression and clinical outcome assessment (assessed by change from baseline on the Performance of Upper Limb version 2) in non-ambulatory patients has not been established.1
Assessment of ELEVIDYS micro-dystrophin levels can be meaningfully influenced by differences in sample processing, analytical technique, reference materials, and quantitation methodologies. Therefore, valid comparisons of ELEVIDYS micro-dystrophin measurements obtained from different assays cannot be made.1

Impact on motor function
— AMBULATORY PATIENTS —
Patients treated with ELEVIDYS scored higher on the NSAA, compared with placebo. While results favored ELEVIDYS, they did not reach statistical significance.1
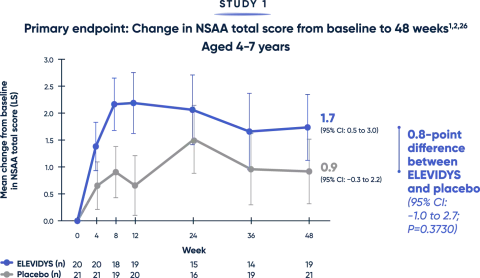
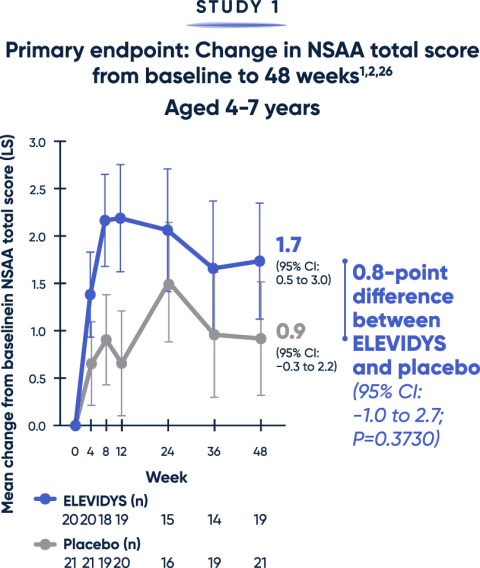
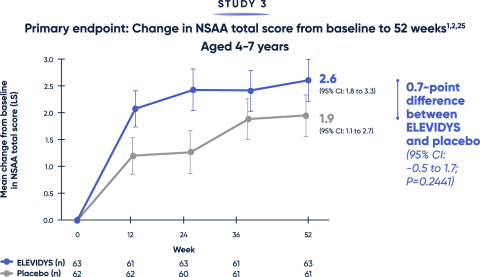
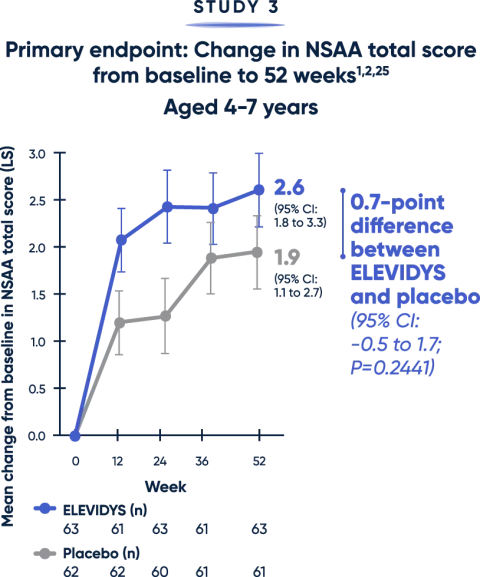
The NSAA provides a broad assessment of physical function using a 3-point scoring method (0=inability, 1=ability with assistance, 2=complete independence). Scoring may limit the sensitivity of NSAA to changes over short time spans.12
LS=least squares; NSAA=North Star Ambulatory Assessment.
Patients treated with ELEVIDYS demonstrated a clinically relevant change in the ability to rise from the floor and walk/run compared with placebo2,25
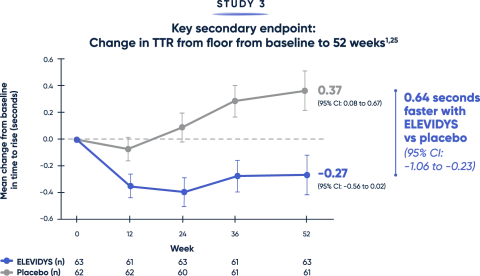
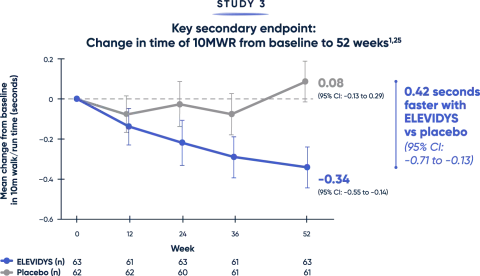
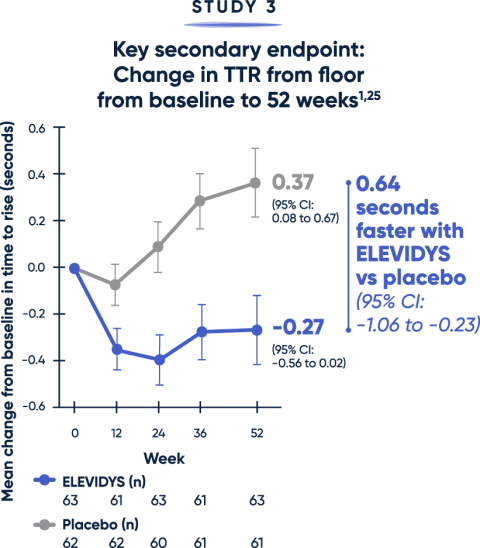
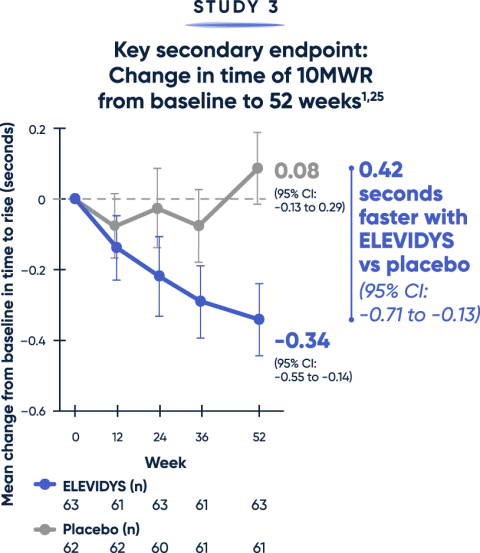
Both the TTR from floor (>5 seconds) and time to complete the 10MWR test (>10 seconds) are key clinical predictors of decline.20,21
MWR=meter walk/run; TTR=time to rise.
Totality of evidence confirms a clinically relevant treatment benefit1
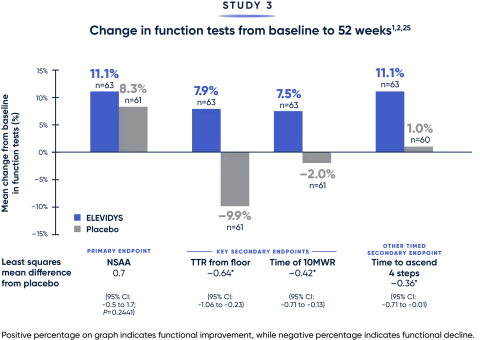
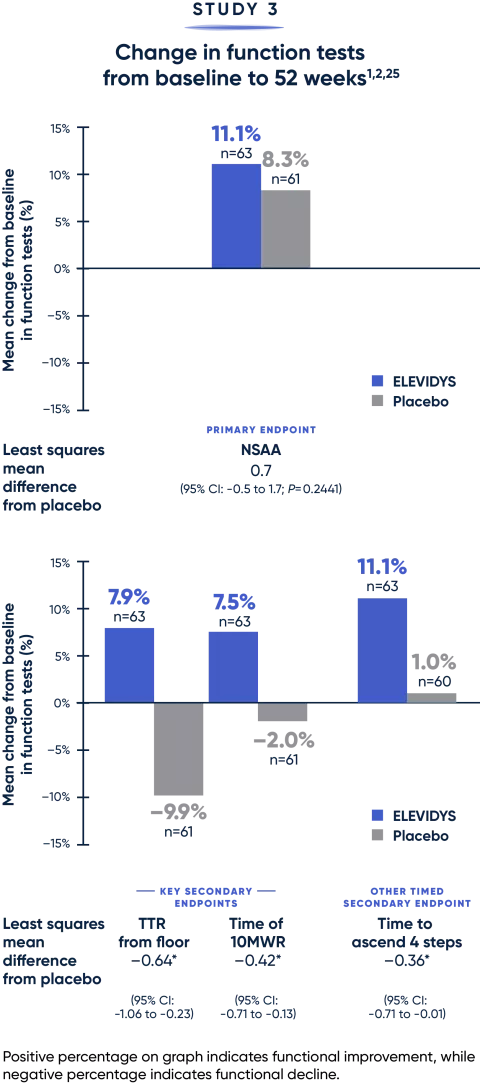
Results favored ELEVIDYS across critical measures of motor function.
*Measured in seconds.
MWR=meter walk/run; NSAA=North Star Ambulatory Assessment; TTR=time to rise.